- Startseite
- Forschung
- AG Neurale Stammzellen
- AG Altern & Demenz
- AG Bewegungsstörungen & Kognition
- AG Bewegungsstörungen & Tiefe Hirnstimulation
- AG Epileptologie
- AG Experimentelle Neuroimmunologie
- AG Klinische Neuroimmunologie
- AG Kognitive Neurologie
- AG Network Plasticity
- AG Neurale Stammzellen
- AG Neuroimaging & Neuroengineering
- AG Neuroinflammation & neuromuskuläre Erkrankungen
- AG Neuromodulation & Neurorehabilitation
- AG Neuroonkologie
- AG Neuropsychologie
- AG Prodromalstadien und Schlaf bei Bewegungsstörungen
- AG Sensomotorische Adaptation
- AG Zerebrovaskuläre Erkrankungen
- Köln-Jülich Biobank
AG Neurale Stammzellen
Die Entdeckung neuraler Stammzellen im adulten Gehirn vor einigen Jahrzehnten hat immenses wissenschaftliches Interesse geweckt und ist mit der Hoffnung verbunden, neurologische Erkrankungen bald besser behandeln zu können. Die endogenen Stammzellen werden durch Schädigungen verschiedener Art zur Proliferation angeregt und tragen zur Regeneration bei. Dabei erreichen sie zwar nur ansatzweise einen Ersatz untergegangener Neurone, sondern ihre Wirkung entfaltet sich vor allem durch andere pleiotrope Effekte wie Neuroprotektion und Immunmodulation. Allerdings ist das intrinsische Potential der neuralen Stammzellen für den Ausgleich akuter Schäden häufig nicht ausreichend. Die AG Neurale Stammzellen versucht aufzuklären, wie diese endogenen neuralen Stammzellen effektiver mobilisiert werden können, um Regeneration im Gehirn nach verschiedenartigen Schädigungen zu verbessern. Hierzu sondieren wir sowohl pharmakologische als auch nicht-pharmakologische Behandlungsansätze, etwa die nicht-invasive Hirnstimulation.
The discovery of neural stem cells in the adult brain a few decades ago evoked immense scientific interest and gave rise to a hope for novel treatments in neurology. Endogenous neural stem cells are stimulated to proliferate by various types of damage and contribute to regeneration. However, they do not (only) attempt this by generating new neurons, but rather by exerting pleiotropic effects such as neuroprotection and immune modulation. However, the intrinsic potential of endogenous neural stem cells to compensate for acute damage is often insufficient. Our research group aims to enhance mobilization of endogenous neural stem cells in order to improve regeneration in the brain after various types of damage. To this end, we are exploring both pharmacological and non-pharmacological treatment approaches, such as non-invasive brain stimulation.
Projects
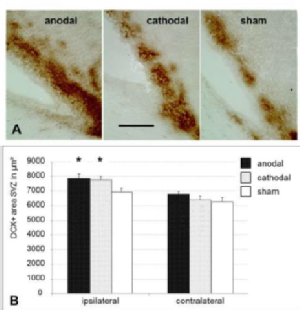
Electrical stimulation of the brain through the intact skull with weak direct currents (transcranial direct current stimulation; tDCS) is a non-invasive method of brain stimulation that is harmless to humans. This subliminal stimulation does not induce neuronal depolarization but rather changes the excitability of the respective cortical region. In addition to these immediate effects, effects that outlast the stimulation (after-effects) are observed. In the last years, we have characterized the previously unknown effects of tDCS on various cell populations in the brain. Interestingly, we observed effects on endogenous neural stem cells as well as on microglia. On the functional level, tDCS accelerates motor recovery from ischemic stroke, an observation we intend to further harness by advancing and optimizing tDCS protocols based on our experimental findings.
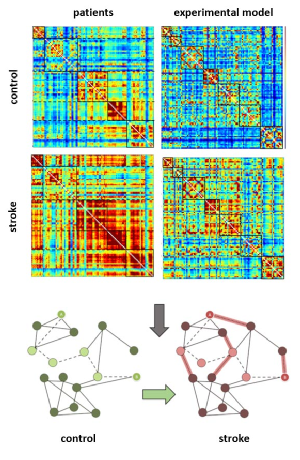
Ischemic stroke does not only affect the primarily hypoxic tissue in the infarct core, but leads to widespread secondary damage. Neuroinflammation sustained by both brain-resident microglia as well as by recruitment of hematogenous cells such as granulocytes, T cells, and monocytes/macrophages is initially aimed at containing the lesion, e.g. by phagocytosing debris. Moreover, immune cells attract endogenous neural stem cells to facilitate regeneration. However, insufficient termination of neuroinflammatory processes leads to serious secondary damage of viable brain tissue, both in the peri-infarct zone as well as in remote regions such as the thalamus. Over the past years, we have extensively characterized the spatio-temporal patterns of neuroinflammation, the recruitment of endogenous neural stem cells, and the multi-facetted interactions between stem cells and microglia, both in vitro and in vivo. Beyond its consequences on the cellular level, ischemic stroke relevantly affects functional neuronal networks, i.e., the functional connectivity between different brain regions. Using resting-state functional magnetic resonance imaging (rs-fMRI), we are able to compare the network changes in various experimental stroke models with human stroke, and find common signatures. These techniques allow us to now evaluate novel treatment options for stroke in a translational setting.
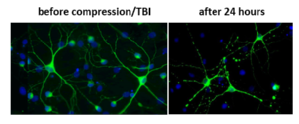
Traumatic brain damage is a significant cause of permanent impairment and reduction in quality of life. In particular, mild but repeated damage in the sense of a commotio cerebri (or concussion), for example in contact sports, can lead to persistent symptoms with neurodegenerative secondary consequences that sometimes progress over years (e.g. chronic traumatic encephalopathy) and cause significant impairments of motor control and cognition. In close collaboration with the Forschungszentrum Jülich (Mechanobiology, IBI-2), we have characterized the influence of mechanical stimuli on cerebral cell groups, including neural stem cells, neurons, and microglia. Based on this, we recently established both a cell culture model of traumatic damage to brain cells based on uniaxial compression, as well as an experimental in vivo model of mild traumatic brain injury. Moreover, we founded the Concussion Center Rheinland to improve medical care for professional and recreational athletes in contact sports. In our current projects, we aim for a better understanding of the effects of mild traumatic brain injury on both the cellular- as well as on the network level.
2019 - 2021
- Walter HL, Pikhovych A, Endepols H, Rotthues S, Bärmann J, Backes H, Hoehn M, Wiedermann D, Neumaier B, Fink GR, Rueger MA, Schroeter M (2022). Transcranial-Direct-Current-Stimulation Accelerates Motor Recovery After Cortical Infarction in Mice: The Interplay of Structural Cellular Responses and Functional Recovery. Neurorehabil Neural Repair; 36 (10-11): 701-714.
- Blaschke S, Hensel L, Minassian A, Vlachakis S, Tscherpel C, Vay SU, Rabenstein M, Schroeter M, Fink GR, Hoehn M, Grefkes C, Rueger MA (2021). Translating functional connectivity after stroke: fMRI detects comparable network changes in mice and humans. Stroke; 52(9): 2948-2960.
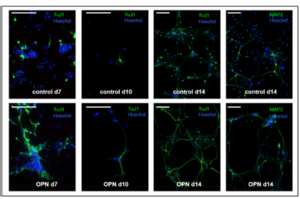
- Vay SU, Olschewski DN, Petereit H, Lange F, Nazarzadeh N, Gross E, Rabenstein M, Blaschke S, Fink GR, Schroeter M, Rueger MA (2021). Osteopontin regulates proliferation, migration, and survival of astrocytes depending on their activation phenotype. J Neurosci Res; 99(11): 2822-2843.
- Schlusche AK, Vay SU, Kleinenkuhnen N, Sandke S, Campos-Martin R, Florio M, Huttner W, Tresch A, Roeper J, Rueger MA, Jakovcevski I, Stockebrand M, Isbrandt D (2021). Developmental HCN channelopathy results in decreased neural progenitor proliferation and microcephaly in mice. Proc Natl Acad Sci USA; 118(35): e2009393118.
- Abraham JA, Blaschke S, Tarazi S, Dreissen G, Vay SU, Schroeter M, Fink GR, Merkel R, Rueger MA, Hoffmann B (2021). NSCs under strain – Unraveling the mechanoprotective role of differentiating astrocytes in a cyclically stretched coculture with differentiating neurons. Front Cell Neurosci; 15: 706585.
- Blaschke SJ, Demir S, König A, Abraham JA, Vay SU, Rabenstein M, Olschewski DN, Hoffmann C, Hoffmann M, Hersch N, Merkel R, Hoffmann B, Schroeter M, Fink GR, Rueger MA (2020). Substrate Elasticity Exerts Functional Effects on Primary Microglia. Front Cell Neurosci;14: 590500.
- Vay SU, Flitsch LJ, Rabenstein M, Monière H, Jakovcevski I, Andjus P, Bijelic D, Blaschke S, Walter HL, Fink GR, Schroeter M, Rueger MA (2020). The impact of hyperpolarization-activated cyclic nucleotide-gated (HCN) and voltage-gated potassium KCNQ/Kv7 channels on primary microglia function. J Neuroinflammation;17(1):100.
- Rabenstein M, Vay SU, Blaschke S, Walter HL, Ladwig A, Fink GR, Rueger MA, Schroeter M (2020). Crosstalk between stressed brain cells: direct and indirect effects of ischemia and aglycemia on microglia. J Neuroinflammation;17(1):33.
- Rabenstein M, Unverricht-Yeboah M, Keuters MH, Pikhovych A, Hucklenbroich J, Vay SU, Blaschke S, Ladwig A, Walter HL, Beiderbeck M, Fink GR, Schroeter M, Kriehuber R, Rueger MA (2019). Transcranial Current Stimulation Alters the Expression of Immune-Mediating Genes. Front Cell Neurosci. 2019;13: 461.
- Blaschke S, Vay SU, Pallast N, Rabenstein M, Abraham JA, Linnartz C, Hoffmann M, Hersch N, Merkel R, Hoffmann B, Fink GR, Rueger MA (2019). Substrate elasticity induces quiescence and promotes neurogenesis of primary neural stem cells - a biophysical in vitro model of the physiological cerebral milieu. J Tissue Eng Regen Med; 13(6): 960-972.
- Abraham JA, Linnartz C, Dreissen G, Springer R, Blaschke S, Rueger MA, Fink GR, Hoffmann B, Merkel R (2019). Directing Neuronal Outgrowth and Network Formation of Rat Cortical Neurons by Cyclic Substrate Stretch. Langmuir; 35(23): 7423-7431.